Tag: bioequivalence
Bioequivalence for Inhalers, Patches, and Injections: What Generic Drugs Must Prove to Work the Same
Bioequivalence for inhalers, patches, and injections isn't about matching blood levels-it's about ensuring the drug reaches the right place the same way. Learn why these generics are harder to approve, cost millions more, and still save lives.
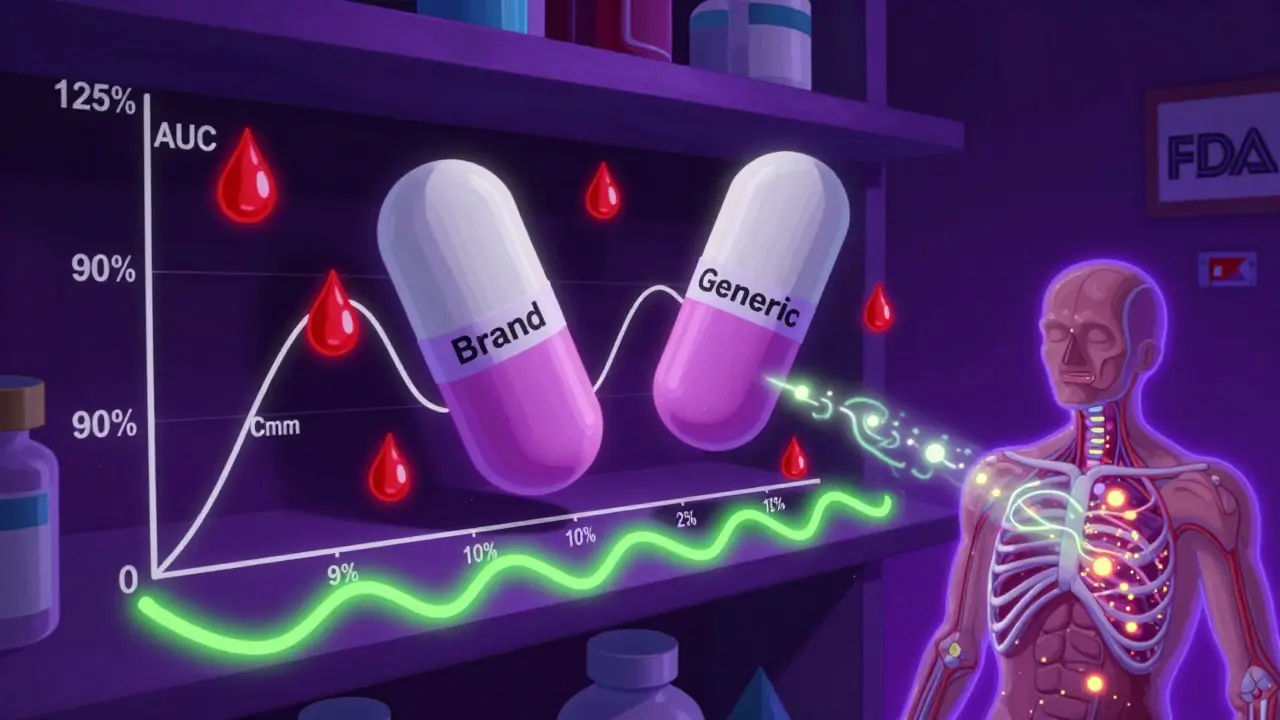
The 80-125% Rule: Understanding Bioequivalence Confidence Intervals in Generic Drugs
The 80-125% rule ensures generic drugs are as safe and effective as brand-name versions by measuring how much of the drug enters your bloodstream. It's based on statistical confidence intervals, not pill content.
Medical Education on Generics: Do Doctors Learn Equivalence?
Despite generics making up 90% of prescriptions, most doctors receive little training on bioequivalence. This article explores why physicians still doubt generic drugs and what medical education must change to close the knowledge-behavior gap.
Clinical Outcomes Data: What Studies Tell Providers About Generics
Clinical outcomes data shows generic drugs are just as effective and safe as brand-name medications for most conditions. Providers can confidently prescribe generics to improve adherence and reduce costs without compromising care.