When you pick up a generic pill at the pharmacy, you might wonder: is this really the same as the brand-name version? The answer lies in something called the 80-125% rule-a quiet but powerful standard that ensures generic drugs work just like their brand-name counterparts. It’s not about how much active ingredient is in the tablet. It’s not about price. It’s about what happens inside your body after you swallow it.
What the 80-125% Rule Actually Means
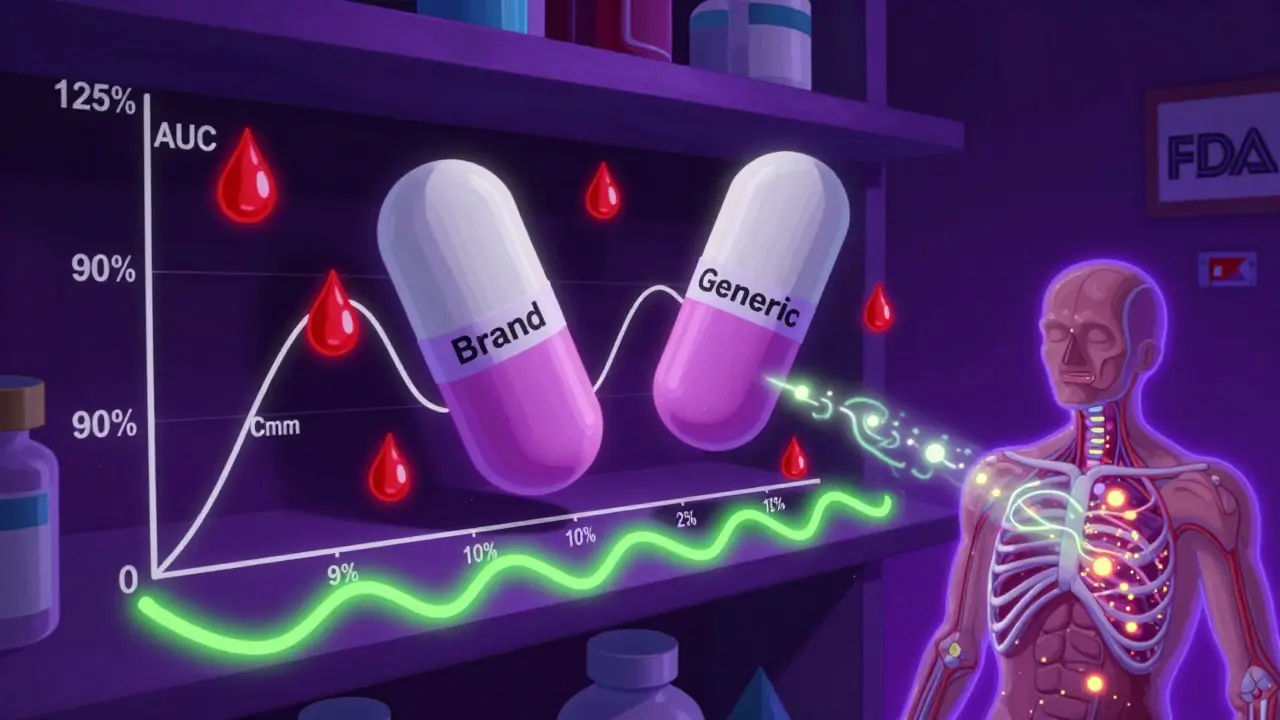
Many people think the 80-125% rule means generic drugs can contain anywhere from 80% to 125% of the active ingredient compared to the brand. That’s wrong. The rule doesn’t refer to the amount of drug in the pill. It refers to how much of that drug actually gets into your bloodstream-and how fast. Here’s how it works: regulators measure two key things after someone takes a drug-Area Under the Curve (AUC) and maximum concentration (Cmax). AUC tells you the total amount of drug absorbed over time. Cmax tells you how quickly it reaches its peak level. These numbers are measured in clinical studies with healthy volunteers, using blood samples taken over several hours. The 80-125% rule says that the 90% confidence interval of the ratio between the generic and brand-name drug’s AUC and Cmax must fall entirely within that range. That’s not a 20% margin of error-it’s a statistical boundary. The data is log-transformed because drug absorption doesn’t follow a normal curve; it follows a log-normal one. On that transformed scale, 80% and 125% are symmetrical around 100%, making the math cleaner and more accurate. This isn’t a guess. It’s based on decades of clinical data. In the 1980s, the FDA held hearings with top pharmacologists who concluded that differences under 20% in drug exposure were unlikely to affect how a patient responds. Since then, over 14,000 generic drugs in the U.S. have been approved using this standard-and post-market surveillance shows fewer than 0.4% needed changes due to bioequivalence issues.Why a 90% Confidence Interval? Not 95%
You’ve probably heard of 95% confidence intervals in other studies. Why is it 90% here? Because the goal isn’t to prove the drugs are identical. It’s to prove they’re close enough to be safe and effective. A 90% confidence interval allows for a 5% chance the true ratio is below 80% and a 5% chance it’s above 125%. That’s a 10% total risk of being outside the acceptable range. That’s considered acceptable because the consequences of small differences in absorption are minimal for most drugs. It’s a balance between statistical rigor and real-world safety. If you used a 95% confidence interval, you’d need much larger studies, more participants, and higher costs. For a generic drug maker, that could mean adding millions to development costs. The 90% CI strikes the right balance: it’s strict enough to protect patients, flexible enough to keep generics affordable.When the Rule Doesn’t Apply-And What Happens Instead
The 80-125% rule is the default, but it’s not universal. Some drugs need tighter limits. Others need looser ones. Take warfarin or levothyroxine. These are narrow therapeutic index drugs-tiny changes in blood levels can cause serious side effects or treatment failure. For these, regulators like the FDA now require a tighter range: 90-111%. That’s because even a 10% difference in exposure could mean the difference between a clot and a stroke, or between feeling fine and feeling exhausted. Then there are highly variable drugs-like certain antibiotics or epilepsy medications-where the same person’s absorption can jump around a lot from day to day. For these, the European Medicines Agency and FDA use something called scaled average bioequivalence (SABE). Instead of a fixed 80-125%, the range expands based on how much the reference drug varies in the body. In extreme cases, the range can stretch to 69.84-143.19% for Cmax. This isn’t a loophole-it’s a smarter way to handle drugs that naturally behave unpredictably. And for complex products-like inhalers, topical creams, or extended-release pills-the rules are still evolving. The FDA launched its Complex Generics Initiative in 2018 with $35 million to develop better methods. These aren’t simple pills you swallow. They rely on delivery systems that are hard to copy exactly. That’s why some generics for these drugs still need clinical outcome studies instead of just blood tests.
Common Misconceptions-And Why They Matter
A 2022 survey found that 63% of community pharmacists thought the 80-125% rule meant generics had less active ingredient. That’s a dangerous misunderstanding. In reality, both brand and generic pills must contain 95-105% of the labeled amount of active drug. The difference isn’t in the tablet-it’s in how your body handles it. Patients often worry. On forums like Drugs.com, people report switching to generics and feeling “different.” But when researchers dig into those reports, only a small fraction are linked to bioequivalence. Most are due to inactive ingredients-fillers, dyes, or coatings-that affect how fast the pill dissolves or how it tastes. Or they’re psychological. If you’ve been on a brand for years, switching-even to an identical drug-can trigger anxiety. The American Epilepsy Society has warned that for some anti-seizure drugs, even small changes in absorption could affect seizure control. That’s why neurologists often prefer to stick with one version. But again, this isn’t because generics fail the 80-125% rule. It’s because those drugs sit right on the edge of safety. The rule works for most drugs. For a few, we need extra caution.How Bioequivalence Studies Are Done
A typical bioequivalence study involves 24 to 36 healthy volunteers. They’re randomly assigned to take either the brand or generic drug first, then switch after a washout period. Blood is drawn every 15 to 30 minutes for up to 72 hours. The data is log-transformed, and the geometric mean ratio is calculated. The 90% confidence interval is then checked against 80-125%. It sounds simple. But it’s not. A single mistake-like not fasting properly before dosing, or mishandling the lab samples-can ruin the whole study. Outliers? If more than 20% of data points are flagged, the study must be justified. High variability? You need more participants-sometimes 50 to 100. Food effects? You need a separate arm of the study to test the drug with a meal. And it’s expensive. A single bioequivalence study costs $2 million to $5 million and takes 18 to 24 months. That’s why generics are cheaper than brand drugs-not because they’re low quality, but because they don’t need to repeat the original 10-year clinical trial program.





Comments
Alexandra Enns
January 25, 2026 AT 12:49 PMLet me stop you right there - this 80-125% rule is just corporate propaganda wrapped in stats. The FDA is in bed with Big Pharma. You think they’d let a $5 pill be truly equivalent? Ha. I’ve seen people crash after switching generics. They don’t test on real patients with real conditions. They test on healthy college kids who’ve never taken a pill in their life. And then they act like it’s science. It’s not. It’s a scam to make drugs cheaper for insurers. You’re being lied to.
Marie-Pier D.
January 26, 2026 AT 13:44 PMWow, this is actually one of the clearest explanations I’ve ever read about bioequivalence 😊 I used to worry every time I switched to generic, especially for my thyroid med. But now I get it - it’s not about the pill, it’s about what your body does with it. Thank you for breaking it down like this. You just made my anxiety disappear 🤗
Chloe Hadland
January 27, 2026 AT 07:36 AMthis is so cool i never realized how much math goes into generics i always just thought they were cheaper copies but turns out its like a whole science experiment in my medicine cabinet
Vatsal Patel
January 28, 2026 AT 08:00 AMSo you’re telling me the same drug that costs $50 is magically the same as $5 because some statisticians decided 90% CI is "good enough"? How quaint. The real question is: why do we even trust regulators who let Big Pharma write the rules? You think they care about you? They care about profit margins. This isn’t science - it’s accounting dressed in a lab coat.
Sharon Biggins
January 28, 2026 AT 13:03 PMi just wanted to say thank you for writing this i had no idea how complex this was and now i feel way less scared about switching to generics i used to think i was getting a second rate version but now i get it its like buying the same car but from a different factory with the same engine
John McGuirk
January 29, 2026 AT 00:41 AMThey’re hiding something. Why 90% and not 95%? Why not publish every single study? Why do the same generics sometimes work and sometimes don’t? There’s a pattern. I’ve seen people go into seizures after switching. The FDA doesn’t track it. They bury it. And you think this is safe? Wake up. This is how they control the masses. Cheap drugs. Quiet deaths. No questions asked.
Michael Camilleri
January 29, 2026 AT 00:47 AMpeople dont get it the 80-125 rule is just a loophole so big pharma can keep making money off brand name drugs while pretending generics are the same but theyre not the same at all the fillers are different the absorption rates vary and if you have a chronic condition you know this isnt just math its your life
lorraine england
January 29, 2026 AT 14:14 PMI love how you explained the log-transformation part - that always confused me. And wow, 14,000 generics approved with under 0.4% issues? That’s actually pretty wild. I used to be super skeptical too, but now I’m like… maybe this system actually works? 😅
Darren Links
January 29, 2026 AT 18:12 PMCanada’s doing it right. We have the same rule, but we also have stricter post-market monitoring. You think the U.S. tracks adverse events? Nah. We do. And guess what? The data backs this up. The 80-125% rule isn’t magic - it’s evidence. Stop being a conspiracy nut and look at the numbers. Real people are getting cheaper meds because of this. Not everyone’s out to get you.
Kevin Waters
January 30, 2026 AT 10:08 AMJust wanted to add - the SABE method for highly variable drugs is genius. It’s not letting loopholes through, it’s adapting to biology. Some drugs just behave weirdly in people. Forcing a fixed range on them is like trying to measure wind speed with a ruler. This isn’t cutting corners - it’s being smart. Kudos to the scientists who built this.
Kat Peterson
January 30, 2026 AT 11:46 AMOMG I JUST REALIZED THIS IS WHY MY ANTI-SEIZURE MEDS FEEL DIFFERENT 😭 I SWITCHED TO GENERIC AND FELT LIKE A ZOMBIE FOR TWO WEEKS. IT WASN’T ME - IT WAS THE FILLERS. I’M GOING BACK TO BRAND. NOBODY TOLD ME THIS WAS A THING. THIS POST CHANGED MY LIFE 🙏💖