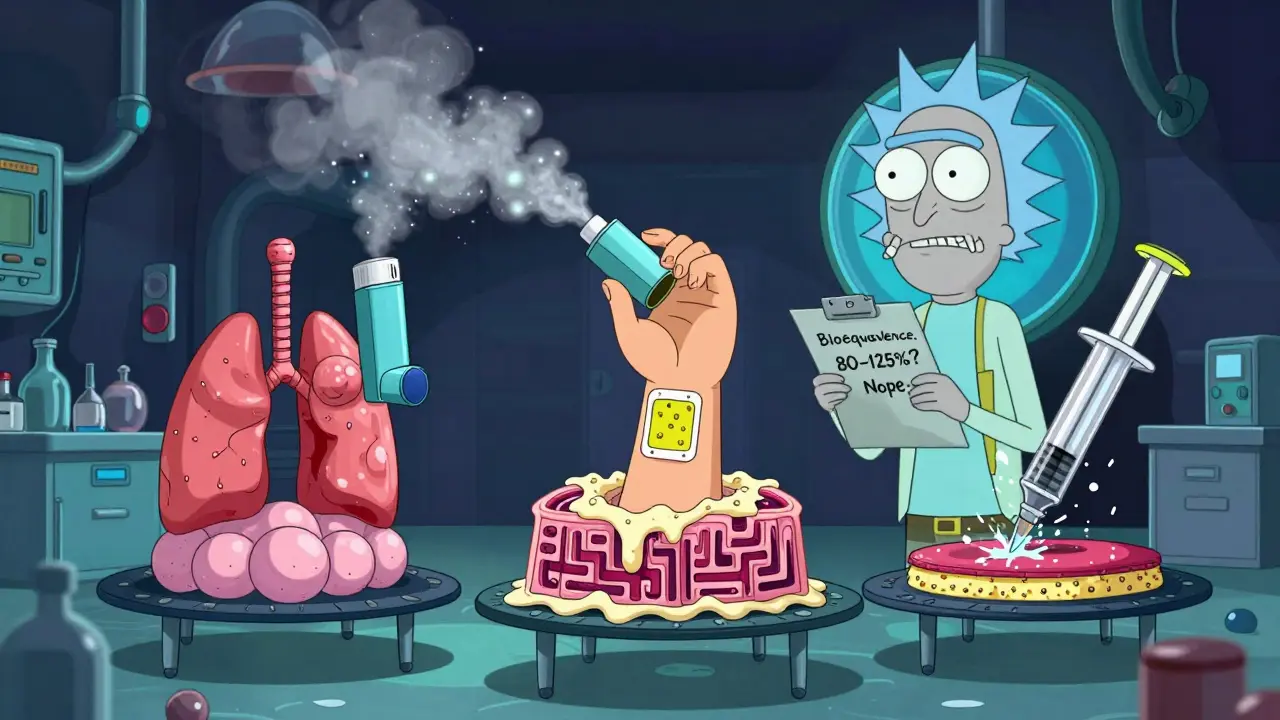
When you pick up a generic inhaler, patch, or injection, you expect it to work just like the brand-name version. But here’s the catch: bioequivalence for these delivery systems isn’t just about the drug amount. It’s about how, where, and when that drug gets delivered inside your body. And that’s where things get complicated.
Why Bioequivalence Isn’t the Same for Inhalers, Patches, and Injections
For a pill, bioequivalence is straightforward. You measure how fast and how much of the drug enters your bloodstream. If the generic’s peak concentration (Cmax) and total exposure (AUC) fall within 80-125% of the brand, it’s approved. Simple.
But that doesn’t work for an inhaler. The drug isn’t meant to enter your blood-it’s meant to land in your lungs. A generic albuterol inhaler might release the same amount of drug, but if the particle size is off by just 0.5 micrometers, most of it hits your throat instead of your airways. That’s not bioequivalent. It’s ineffective.
Same with patches. A nicotine patch might release the same total dose over 24 hours, but if the adhesive doesn’t stick the same way, or if the drug leaks unevenly through your skin, your blood levels could spike or drop unpredictably. For injections, especially ones with nanoparticles or liposomes, the drug’s physical structure matters more than the chemical formula. If the particle size changes, the body absorbs it differently-sometimes dangerously so.
The FDA calls this the "site of drug action." For oral drugs, it’s the bloodstream. For inhalers, it’s the lung surface. For patches, it’s the skin layers. For some injectables, it’s the tissue around the injection site. You can’t measure that with a blood test alone.
How Inhalers Are Tested for Bioequivalence
Testing an inhaler isn’t just about how much drug it delivers. It’s about how it delivers it.
- Particle size: 90% of particles must be between 1 and 5 micrometers. Too big? They get stuck in your throat. Too small? They get exhaled before reaching your lungs.
- Plume geometry: The spray pattern must match the original. A 2°C difference in plume temperature can change how the drug disperses. The FDA rejected a generic inhaler in 2019 for this exact reason.
- Delivered dose uniformity: Each puff must deliver within 75-125% of the labeled dose. No outliers.
- In vivo testing: For corticosteroid inhalers, they don’t just measure blood levels-they measure lung function. If your FEV1 (a key breathing test) doesn’t improve the same way as with the brand, it fails.
One study found that only 38% of generic inhaler applications get approved-far lower than the 78% approval rate for pills. Why? Because every component matters: the propellant, the valve, the canister pressure, even the plastic casing. A single change in any of these can derail the entire product.
Transdermal Patches: The Slow Burn Challenge
Transdermal patches are designed to release drug slowly over hours or days. That makes bioequivalence tricky. You can’t just compare peak levels because there isn’t one.
- In vitro release rate: The patch must release drug at the same rate at every time point over 24 hours-within 10% of the brand. Not just the total amount, but the curve.
- Skin adhesion: If the patch peels off early, or doesn’t stick evenly, the drug delivery becomes inconsistent. Some generics failed because the adhesive cracked under sweat or movement.
- Residual drug content: After use, the patch should have the same amount of leftover drug. If a generic leaves more drug behind, it means less was absorbed.
- AUC over Cmax: For patches, the total exposure (AUC) is what matters. Cmax is often ignored because it’s not meaningful with sustained release.
Despite the complexity, patches have a 52% approval rate-higher than inhalers. Why? Because the science is better understood. We’ve had nicotine and fentanyl patches for decades. We know exactly how they behave on skin. But newer patches, like those for hormones or pain relievers, still face high failure rates.

Injectables: When the Drug’s Shape Matters
Not all injections are the same. A simple saline solution? Easy. A liposomal injection like Doxil? That’s a whole different ballgame.
- Particle size: Must be within 10% of the brand. A 100nm particle vs. 110nm changes how the body clears the drug.
- Zeta potential: The surface charge must be within 5mV. It affects how the particles interact with cells.
- Polydispersity index: Must be under 0.2. This measures how uniform the particle sizes are. A high index means a messy, inconsistent mix.
- In vitro release profile: The drug must release at the same rate over time-whether it’s 1 hour or 10 hours.
For drugs with a narrow therapeutic index-like enoxaparin (Lovenox)-the limits tighten to 90-111%. One percent too much, and you risk bleeding. One percent too little, and you risk clots.
The FDA rejected a generic version of Bydureon BCise in 2021-not because of the drug, but because the auto-injector mechanism delivered the dose 0.2 seconds slower. That tiny delay changed how the drug spread in tissue. The sponsor lost $45 million.
Why Approval Rates Are So Low-and Why It Matters
Here’s the hard truth: developing a generic inhaler, patch, or injectable costs $25-40 million. For a pill? $5-10 million. The timeline? 36-48 months versus 18-24.
And even then, approval isn’t guaranteed. The FDA’s Office of Generic Drugs approved 788 generics in 2022-but only 45 were complex products. That’s less than 6%.
Why does this matter? Because patients pay more. Special delivery system generics make up 30% of prescriptions but only 15% of the generic market by value. Why? Because manufacturers can’t afford to risk the investment unless they’re sure it’ll pass. So fewer companies try. That means less competition. And higher prices.
Success stories exist. Teva’s generic ProAir RespiClick captured 12% of the market within 18 months after proving lung deposition with scintigraphy imaging. But those are rare. Most companies give up after one or two failed attempts.

What’s Changing-and What’s Still Broken
Regulators know the system is flawed. The FDA, EMA, and WHO now use a "totality of evidence" approach. That means they look at:
- Physicochemical properties
- In vitro performance
- Pharmacokinetic data
- Pharmacodynamic outcomes
- Even patient training materials (yes, for inhalers)
New tools are helping. Physiologically-based pharmacokinetic (PBPK) modeling is now used in 65% of complex generic submissions-up from 22% in 2018. It simulates how the drug behaves in the body based on real human anatomy, not just blood samples.
But gaps remain. We still don’t have good in vitro-in vivo correlations (IVIVC) for most inhalers. Only 35% of companies succeed in building them. And for newer devices-like prefilled auto-injectors or smart inhalers with sensors-we’re flying blind.
There’s also "biocreep." Imagine a brand-name inhaler. Then a first generic. Then a second. Each passes bioequivalence. But over time, small changes add up. A 3% difference here, a 2% difference there. Eventually, the last generic might not work like the original. No one tests that. No one tracks it.
Who’s Doing It Right-and Who’s Struggling
Only 28 companies have approved complex generics. Teva leads with 14, Mylan with 9, Sandoz with 8. They have teams of 50+ scientists, labs with cascade impactors ($300,000 each), and regulatory experts who live in the FDA’s guidance documents.
Small companies? They’re out. The cost and complexity are too high. The FDA’s Complex Generic Drug Product Development program has helped 42 small businesses since 2018, but that’s a drop in the ocean.
Industry feedback? Mixed. A formulation scientist spent 42 months and $32 million on a generic insulin glargine product. "We had 17 formulation iterations just to get the particle size right," he said. Another reported their generic albuterol was rejected because the plume temperature was 2°C higher. "It felt like we were being punished for physics."
But most agree: this isn’t about slowing generics. It’s about saving lives. A poorly delivered inhaler can mean a trip to the ER. A patch that doesn’t stick can mean withdrawal symptoms. A misdelivered injection can mean a stroke.
What Patients Should Know
You don’t need to understand particle size or zeta potential. But you should know this:
- If your generic inhaler doesn’t seem to work like the brand, tell your doctor. It might not be bioequivalent.
- Don’t assume all patches are the same. If one starts peeling or feels different, ask if it’s the same formulation.
- Complex injectables aren’t interchangeable without proof. Ask your pharmacist if the generic has been tested for the same delivery profile.
Regulators are trying. Science is improving. But the system is still fragile. The goal isn’t just to approve generics-it’s to ensure they work the same. And for these delivery systems, that’s not easy. It’s not even close to simple.
Why can’t we just use blood tests for inhalers and patches like we do for pills?
Because the drug isn’t meant to enter the bloodstream. For inhalers, the drug needs to land in the lungs. For patches, it needs to pass through the skin slowly. Blood tests measure systemic exposure, but they don’t tell you if the drug reached the right place at the right time. A generic inhaler might show the same blood levels as the brand-but deliver 40% less drug to the lungs. That’s not equivalent. That’s dangerous.
Are generic inhalers and patches safe?
Yes-if they’ve passed the full bioequivalence testing. Approved generics go through the same rigorous review as brand-name products. But not all generics make it. Those that do are just as safe. The problem is, many never get approved because they fail the complex tests. So the ones you find on the shelf are safe. The ones that never made it? We don’t see them.
Why are complex generics so expensive to develop?
Because you’re not just copying a chemical. You’re copying a delivery system. That means matching particle size, spray pattern, adhesive strength, release rate, device mechanics, and more-all within tiny tolerances. Each test requires specialized equipment, hundreds of hours of testing, and expert analysis. A single inhaler study can cost $5 million just in testing. Add in failed attempts, regulatory delays, and legal costs, and you’re looking at $30 million or more.
Do all countries have the same bioequivalence rules?
No. The FDA and EMA have detailed, product-specific guidance, but many countries still rely on outdated standards. A generic approved in the U.S. might not be accepted in India or Brazil because they lack the infrastructure to test complex delivery systems. This creates global disparities in access. The Global Bioequivalence Harmonization Initiative is trying to fix this, but progress is slow.
Will bioequivalence standards get easier for generics?
Not easier-but smarter. The future lies in better tools: PBPK modeling, advanced imaging, AI-driven in vitro testing, and real-world data. We’re moving away from one-size-fits-all bioequivalence. Instead, we’re building custom pathways for each delivery system. That means more accurate approvals, not more approvals. Quality over quantity.