Every year, hundreds of thousands of people in the UK and around the world end up in hospital because of unexpected side effects from medications they were prescribed. These aren’t rare accidents-they’re adverse drug reactions (ADRs), and many of them are completely preventable. The problem isn’t that doctors are careless. It’s that we’ve been treating drugs like one-size-fits-all tools, when in reality, your genes decide how your body handles them.
Why Your Genes Matter When You Take a Pill
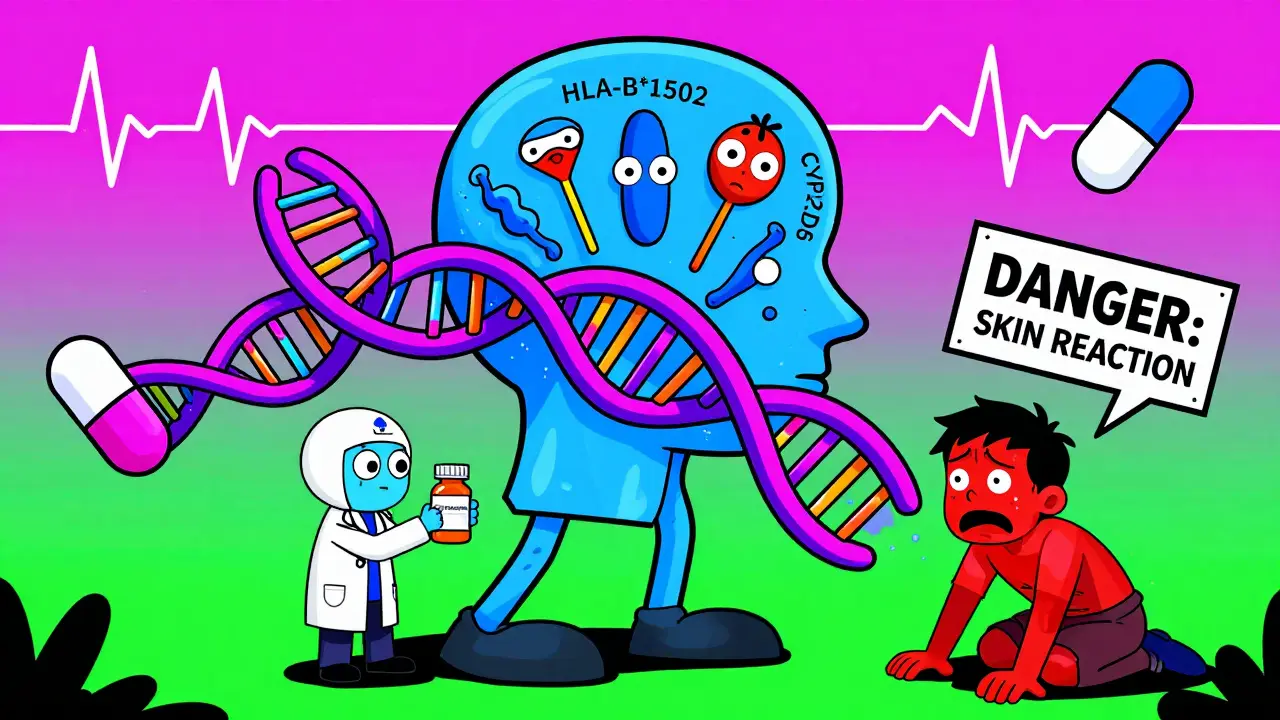
You might not think about it, but the way your body breaks down medicine is coded in your DNA. Two people can take the same dose of the same drug, and one might feel fine while the other gets sick-sometimes dangerously so. That’s not luck. It’s genetics. Take carbamazepine, a common drug for epilepsy and nerve pain. For some people, especially those of Asian descent, this drug can trigger a life-threatening skin reaction called Stevens-Johnson syndrome. But here’s the key: if you carry a specific gene variant called HLA-B*1502, your risk jumps from less than 1 in 10,000 to nearly 1 in 10. Testing for that single variant before prescribing the drug cuts that risk by 95%. It’s not just carbamazepine. The same thing happens with:- Azathioprine (used for autoimmune diseases): People with low TPMT enzyme activity can develop severe bone marrow damage. Testing prevents this in 78% of cases.
- Clopidogrel (a blood thinner): If you’re a poor metabolizer of CYP2C19, the drug won’t work. You’re at higher risk of heart attack or stroke-not because the drug failed, but because your genes stopped it from activating.
- Statins (cholesterol drugs): SLCO1B1 variants can cause muscle damage. Knowing your status lets doctors choose a safer alternative.
The Landmark Study That Changed Everything
In 2023, the PREPARE study published its results in The Lancet, and it was the biggest real-world test of pharmacogenetic testing ever done. Nearly 7,000 patients across seven European countries were tested for 12 key genes before starting any new medication. Their results were fed directly into their electronic health records, and doctors got alerts when a drug might be risky. The outcome? A 30% drop in serious adverse drug reactions. That’s not a small improvement. That’s like preventing one hospitalization for every three patients on multiple medications. And the best part? This wasn’t done in a research lab. It was done in regular hospitals, with real doctors, real nurses, and real patients. What made this different from past studies? It was preemptive. Instead of waiting for someone to get sick and then testing, they tested everyone upfront-before any drug was even prescribed. That’s the game-changer. Reactive testing (after an ADR happens) only cuts risk by 15-20%. Preemptive testing? 30%.What Genes Are Tested-and Why
The PREPARE study didn’t test every gene under the sun. It focused on 12 genes that have the strongest, most reliable links to drug reactions. These genes control how your body absorbs, breaks down, or responds to over 100 commonly used drugs. Here’s what matters most:- CYP2D6, CYP2C19, CYP2C9, CYP3A5: These are your liver’s drug-processing enzymes. Variants here tell you if you’re a slow, normal, or ultra-fast metabolizer. Slow metabolizers build up toxic levels. Fast metabolizers clear the drug too quickly-it doesn’t work.
- TPMT, DPYD: These enzymes break down chemotherapy and immune drugs. Low activity = high risk of life-threatening toxicity.
- SLCO1B1: Controls statin uptake in the liver. Bad variant = higher chance of muscle pain and damage.
- HLA-B*1502, HLA-A*3101: These are immune system flags. Carry them, and certain drugs can trigger deadly skin reactions.
- VKORC1: Affects warfarin dosing. Too much = bleeding. Too little = clots.

How It Works in Practice
Imagine you’re a GP. You’re about to prescribe a new antidepressant. Instead of guessing based on symptoms and trial-and-error, you order a pharmacogenetic test. Three days later, your EHR pops up a notification: > “Patient is a CYP2D6 poor metabolizer. Avoid amitriptyline and paroxetine. Consider sertraline or citalopram instead.” That’s not science fiction. That’s happening now in clinics in the UK, the US, and across Europe. The system works because it’s built into the workflow. The doctor doesn’t have to remember a hundred gene-drug pairs. The computer does it for them. This kind of integration is why the University of Florida Health system saw a 75% drop in ADR-related ER visits after implementing preemptive testing. They spent $1.2 million upfront on software, training, and staff time-but saved over $2 million in avoided hospital stays within 18 months.What’s Holding It Back?
The science is solid. The evidence is clear. So why isn’t everyone doing this? One big reason: cost. A full panel test runs between £150 and £350 in the UK. That sounds expensive until you consider the alternative. A single hospital admission for an ADR can cost over £5,000. The NHS spends £500 million a year on ADR-related hospital stays. Preventing just 10% of those would pay for testing every adult in the country. Another problem? Doctors aren’t trained for it. A 2022 survey found only 37% of physicians felt confident interpreting pharmacogenetic results. That’s changing. The Clinical Pharmacogenetics Implementation Consortium (CPIC) now offers free, up-to-date guidelines for 34 gene-drug pairs. And the Dutch Pharmacogenetics Working Group (DPWG) has created clear action plans for when a gene variant is found. There’s also a gap in diversity. Most genetic data comes from people of European descent. If you’re from African, Indigenous, or South Asian backgrounds, the test might miss variants that matter to you. That’s why the NIH and other groups are now actively expanding databases to include underrepresented populations. New variants are being added every year.
Who Benefits the Most?
You might think this is only for people on complex drug regimens. But here’s the truth: the more medications you take, the higher your risk. That’s why these groups see the biggest payoff:- Patients on 5+ medications: Polypharmacy multiplies interaction risks. Testing cuts that chaos.
- Older adults: Metabolism slows with age. Genetic factors make it worse.
- People with mental health conditions: Psychiatric drugs have high ADR rates. A 2024 study showed a 40% drop in side effects after genotype-guided prescribing.
- Cancer patients: Chemotherapy toxicity is a major cause of treatment delays. Testing prevents life-threatening drops in white blood cells.
The Future Is Already Here
The global market for pharmacogenomics is set to hit $22 billion by 2028. The FDA has added 42 new gene-drug pairs to its official list since 2022. The European Commission is investing €150 million to roll out preemptive testing across member states by 2027. New technology is making it cheaper and faster. By 2026, point-of-care tests-like a rapid PCR chip you can run in a doctor’s office-could bring the cost down to under £50 per test. Imagine getting your results before you leave the clinic. And it’s not just about avoiding bad reactions. It’s about finding the right drug the first time. No more months of trying antidepressants that don’t work. No more switching painkillers because one gave you stomach ulcers. Pharmacogenetics turns guesswork into precision.What You Can Do Today
You don’t have to wait for your GP to offer this. If you’re on multiple medications, have had a bad reaction before, or are about to start a new drug-ask about pharmacogenetic testing. Say it clearly: “Is there a genetic test that could help me avoid side effects with this medication?” Some private labs in the UK offer direct-to-consumer panels, but make sure they’re CLIA- or UKAS-accredited. And always discuss results with your doctor. A genetic report isn’t a diagnosis-it’s a tool. If your hospital or clinic doesn’t offer testing yet, ask them why. Demand matters. The PREPARE study showed it works. Now it’s time to make it standard.Medicine is no longer about treating everyone the same. It’s about treating you-the right way, the first time. Your genes are already speaking. It’s time we started listening.





Comments
Stuart Shield
January 6, 2026 AT 00:45 AMMan, I’ve seen this in the NHS-old Mrs. Henderson on five meds, ended up in A&E because her statin turned her legs into jelly. No one thought to check SLCO1B1. If they had, she’d still be gardening. This isn’t sci-fi, it’s basic biology. Why are we still playing Russian roulette with prescriptions?
Susan Arlene
January 7, 2026 AT 21:51 PMso like… my aunt took that antidepressant and just… fell apart? like, crying in the cereal aisle? turns out she’s a slow CYP2D6 metabolizer. no one told her. they just said ‘try another one.’ like its a flavor choice. we’re literally guessing with people’s lives lmao
Mukesh Pareek
January 9, 2026 AT 19:53 PMPharmacogenomics is the logical culmination of precision medicine, leveraging SNP-based pharmacokinetic profiling to mitigate polypharmacy-induced cytochrome P450-mediated metabolic discordance. The PREPARE trial’s statistical power (n=7,000) validates the clinical utility of preemptive genotyping, particularly in high-risk CYP2C19 and TPMT variants. Without standardized genomic integration into EHRs, we’re practicing pharmacological astrology.
Lily Lilyy
January 11, 2026 AT 02:01 AMThis gives me so much hope! 🌟 Imagine a world where no one has to suffer because a doctor didn’t know their genes. We’re not just treating illness anymore-we’re respecting the human body like the incredible, unique machine it is. Thank you for sharing this. Let’s make this standard everywhere!
Joann Absi
January 11, 2026 AT 09:04 AMAMERICA IS THE ONLY COUNTRY THAT DOES THIS RIGHT 😤 I mean, look at the UK-they’re still stuck in the 1980s with ‘guess and hope’ medicine. Meanwhile, Florida hospitals saved $2M?! That’s not a win, that’s a wake-up call to the whole damn world. 🇺🇸✨ #GenesNotGuesswork #YankeeIngenuity
Ashley S
January 13, 2026 AT 02:01 AMSo now we’re gonna test everyone’s DNA before giving them aspirin? Next they’ll be scanning your soul for ‘bad vibes’ before prescribing coffee. This is just corporate greed wrapped in science jargon. I’m not paying £300 so some lab can tell me I’m not a ‘fast metabolizer.’
Indra Triawan
January 13, 2026 AT 06:35 AMEvery time I hear about genes and drugs, I think of my cousin in Mumbai. She took carbamazepine for seizures. One day, her skin started peeling. They didn’t test for HLA-B*1502 because ‘it’s too expensive.’ She spent six months in ICU. Now she can’t even touch sunlight. This isn’t science. It’s a class war. Your DNA doesn’t care if you’re rich or poor-but the system does.
Pavan Vora
January 13, 2026 AT 17:44 PMInteresting… but, uh, I’m from India, and I’m not sure how well these European gene variants apply to South Asian populations… I mean, I’ve read studies… uh… the DPWG guidelines… they’re great… but… I think we need more data… from… um… diverse groups… because, like, our… genetics… are… different…? Maybe? I’m not an expert… but… it seems… important…?
Saylor Frye
January 14, 2026 AT 08:13 AMWow. So you’re telling me we’ve been giving people drugs like they’re Lego bricks? And now we’re just realizing some people’s bodies don’t snap together the same way? Groundbreaking. I’m sure the pharmaceutical lobby is weeping into their champagne flutes.